Diffusion tensor imaging (DTI) and plasma p-tau 181 in Alzheimer’s disease
Document Type : Original Communication
Authors
1 School of Medicine, Iran University of Medical Sciences, Tehran, Iran
2 Neuroscience Research Center (NRC), Iran University of Medical Science, Tehran, Iran
Abstract
Alzheimer’s Disease (AD) is characterized by cognitive impairments and memory difficulties, which cause daily activities, and personal and behavioral problems. In recent years blood-based biomarkers like plasma phosphorylated tau protein at threonine 181 (p tau 181) emerged as new tools and showed a sufficient power in detecting AD patients from healthy people. Here we investigate the correlation between p tau 181 and white matter microstructural changes in AD patients. We add 21 Alzheimer diagnosed patients with baseline plasma p tau level, CSF Amyloidβ, CSF Tau, CSF p Tau, and DTI metrics from the ADNI database. The analysis revealed that the plasma level of p tau 181 could predict changes in MD, RD, AD, and FA parameters in several regions Also, there is a significant association between white matter pathways alteration in different regions with each of the CSF biomarkers. In conclusion, our study results show that plasma p tau 181 levels are associated with microstructural changes in pathogenesis areas of Alzheimer's disease, which enhance this biomarker's diagnostic status. Longitudinal studies are also necessary to prove the efficacy of these biomarkers and predicting role in structural changes.
Keywords
Main Subjects
Introduction
Alzheimer’s disease (AD) is the course of dementia and memory deficits that affect millions of people and are responsible for cognitive and functional decline, mostly in older adults(1, 2). AD is characterized by cognitive impairments and memory difficulties, which cause daily activities, and personal and behavioral problems (1, 3). Neurofibrillary tangles (NFTs), including hyperphosphorylated tau protein (p tau) and elevated extracellular Amyloid β (Aβ) plaques in regions responsible for memory and other cognitive functions in the course of dementia (4, 5). NFTs are typical brain lesions that consist of aggregated and hyperphosphorylated forms of tau protein, which leads to the loss of its ability to binding microtubules and assembled them into paired helical filaments. Intracellular NFTs in regions involved in cognitive functions are associated with cognitive decline by the disruption in axonal transport and neural loss (6, 7).
Researchers investigated Aβ, total Tau and Tau phosphorylated at threonine 181 (p tau 181) in cerebrospinal fluid (CSF), and Positron emission tomography (PET) as biomarkers for AD diagnoses. However, in recent years plasma biomarkers emerged as new tools and showed sufficient power in detecting AD patients from healthy people (8). Blood-based measures showed that p tau 181 might be a reliable biomarker for AD and disease progression (9-11). However, there is limited evidence of the effect of plasma p tau 181 on white matter connections and microstructural changes in AD patients (12-15). We hypothesized plasma p tau 181 level could predict structural brain connections changes in regions that play a role in cognitive performance. We investigated the correlation between p tau 181 in serum and diffusion tensor imaging (DTI) parameters in a cross-sectional study to address this question.
Materials and methods
Data Acquisition
Data used in this article's preparation were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. ADNI participants are recruited from across the United States and Canada. We extracted data from baseline visits of patients from the ADNI-2 and ADNI-3 cohort for whom demographic data, CSF Amyloid β, CSF p tau, CSF total, and plasma p tau 181 tau levels from baseline visits were available. We also extracted the baseline Diffusion tensor imaging (DTI) scan data of all available patients in the cohort. Our observational cross-sectional study consisted of 21 patients with AD, with their baseline plasma p tau 181, CSF Amyloid β, CSF total tau, and CSF p tau levels and their Diffusion tensor imaging (DTI) scan data all acquired at the Banner Alzheimer's Institute (Phoenix, Arizona) and downloaded on December 4, 2018 (http://adni.loni.usc.edu).
In summary, ADNI participants aged between 55 and 90 were all willing to undergo all test procedures, including lumbar punctures at the screening visit and DTI scan. Patients were excluded if they had a high ischemic score in their diffusion-weighted magnetic resonance imaging, a recent change in medications in the four weeks before the study, less than six grades of education or depression. None of the subjects took cholinesterase inhibitors, antidepressant medications with anticholinergic properties, neuroleptic agents, antiparkinsonian drugs, chronic anxiolytics, and sedative-hypnotics diuretics, or were regular narcotic users at the time or within four weeks before the screening visit. AD and MCI patients were allowed to take cholinesterase inhibitors and memantine if the dosing had not changed within four weeks prior to the screening visit. The same drug exclusion criterion was applied for 6- and 12-month follow-ups.
Classification of patients as MCI or AD was based on either of the following criteria: (i) mini-mental state examination (MMSE) score above 24 and under 30 for MCI and higher than 20 and lower than 24 for AD, (ii) clinical dementia rating (CDR) score of 0.5 with memory box score 0.5 for MCI and 0.5 or 1 for AD, or (iii) logical memory II subscale of the Wechsler memory scale below the age categorized cut off values. All patients also fulfilled the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (16).
Plasma p tau 181 Measurements
ADNI samples were analyzed by the Single-Molecule array (Simoa) technique, using an in-house assay developed in the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden. The assay uses a combination of two monoclonal antibodies (Tau12 and AT270) and measures N-terminal to mid-domain forms of P-tau181. Details of the assay can be found in (11). Calibrators were run as duplicates, while plasma samples were measured in singlicate.
CSF biomarkers measurements
Baseline CSF samples were obtained from all subjects in the morning after overnight fasting, as described in the ADNI procedures manual (http://www.adni-info.org/). CSF samples from each site were stored in polypropylene transfer tubes and shipped to the ADNI Biomarker Core laboratory at the University of Pennsylvania Medical Center on dry ice within 1 hour after collection. Aliquots (0.5 mL) were prepared from these samples after thawing, then stored at 80C̊ (17). all CSF Aβ1-42, t-tau, and p-tau181 concentration measurements have been made using the micro-bead-based multiplex immunoassay, the INNO-BIA AlzBio3 RUO test (Fujirebio, Ghent, Belgium) (18), on the Luminex platform.
DTI Imaging Processing
We extracted the results of the DTI ROI analysis from ADNI. For each subject, all raw DWI volumes were aligned to the average b0 image (DTI volume with no diffusion sensitization) using the FSL eddy-correct tool (www.fmrib.ox.ac.uk/fsl) to correct for head motion and eddy current distortions. All extracerebral tissue was roughly removed from the T1-weighted anatomical scans using several software packages, primarily ROBEX, a robust automated brain extraction program trained on manually "skull-stripped" MRI data from (19) and Freesurfer (20). The resulting skull-stripped volumes were visually inspected, and the best one was selected and sometimes further manually edited. Anatomical scans subsequently underwent intensity inhomogeneity normalization using the MNI nu_correct tool (www.bic.mni.mcgill.ca/software/).
Non-brain tissue was also removed from the diffusion-weighted images using the brain.
Extraction Tool (BET) from FSL (21). To align data from different subjects into the same 3D coordinate space, each T1- the weighted anatomical image was linearly aligned to a version of the Colins27 brain template (22) using FSL’s flirt (23) with 6 degrees of freedom to allow translations and rotations in 3D. The Colin27 brain was zero-padded to have a cubic isotropic image size 220x220x220 1mm^3) and then downsampled (110x110x110 2mm^3) to be more similar to the DWI resolution.
To correct for echo-planar imaging (EPI) induced susceptibility artifacts, which can cause distortions at tissue-fluid interfaces, skull-stripped b0 images were linearly aligned to their respective T1-weighted structural scans using FSL’s flirt with 9 degrees of freedom and then elastically registered to their aligned T1 scans using an inverse consistent registration algorithm with a mutual information cost function (24) as described in (25). The resulting 3D deformation fields were then applied to the remaining 41 DWI volumes prior to mapping diffusion parameters. To account for linearly registering the average b0 from the DWI images to the structural T1-weighted scan, a corrected gradient table was calculated.
A single diffusion tensor was modeled at each voxel in the brain from the eddy- and EPI-corrected DWI scans using FSL’s dtifit command, and scalar anisotropy and diffusivity maps were obtained from the resulting diffusion tensor eigenvalues (λ1, λ2, λ3). Fractional anisotropy (FA) was calculated from the standard formula.
We registered the FA image from the JHU DTI atlas (26) to each subject using a previously described mutual information-based elastic registration algorithm(24). We then applied the deformation to the stereotaxic JHU “Eve” WM atlas labels (http://cmrm.med.jhmi.edu/cmrm/atlas/human_data/file/AtlasExplanation2.htm ) using nearest neighbor interpolation to avoid intermixing of labels. This placed the atlas ROIs in the same coordinate space as our DTI maps. We were then able to calculate the average FA and MD within the boundaries of each of the ROI masks for each subject. Of the 56 WM ROIs, we excluded 4 ROIs, the left and right middle cerebellar peduncle, and the pontine crossing tract, as they often fall wholly or partially out of the field of view (FOV). We note that this is also occasionally true of the left and right medial lemniscus, inferior and superior peduncles. We only included non-zero voxels within the FOV in our calculations of mean FA and MD. In addition to the 52 JHU labels, five more ROIs were evaluated: the bilateral fornix, bilateral genu, bilateral body, and bilateral splenium of the corpus callosum and the full corpus callosum, to get full summary measures of the regions.
Tensor-based spatial statistics (27) were also performed, and the mean FA in regions of interest along the skeleton was extracted. TBSS was performed according to protocols outlined by the ENIGMA-DTI group: http://enigma.loni.ucla.edu/wpcontent/uploads/2012/06/ENIGMA_TBSS_protocol.pdf
In short, all subjects were registered to the ENIGMA-DTI template in ICBM space and
standard tbss steps were performed to project individual FA maps onto the skeletonized
ENIGMA-DTI template. ROI extraction was also performed according to the following protocol to extract the mean FA in ROIs along with the skeleton: http://enigma.loni.ucla.edu/wpcontent/uploads/2012/06/ENIGMA_ROI_protocol.pdf.
Cognitive assessments
The patients' cognitive condition is assessed by the Mini-Mental State Exam (MMSE) which is a widely used test of cognitive function among the elderly; it includes tests of orientation, attention, memory, language, and visual-spatial skills. MMSE scores were extracted for each patient from the ADNI Mini-Mental Examination.
Statistical Analyses
We used SPSS16 software for statistical analyses. We investigate the correlation between DTI parameters for each region and CSF biomarkers and the correlation between CSF and plasma biomarkers with each other and with MMSE score, Age, white matter hyperintensity, gray matter volume, white matter volume, and hippocampus volume using single linear regression. Next, we create a multiple linear regression model with age, gender, and plasma p tau 181 level as a covariate and DTI parameters in each region as a dependent variable. Finally, to confirm the result of multiple regression, we used a partial correlation between plasma p tau 181 and microstructural measures that controlled for the effect of age and sex. We examined the reliability of significant regions with a type I error rate set at 0.05.

Result
In an exploratory analysis, we investigated the potential correlation of connectometry values of different brain regions and each patient's characteristics with plasma p tau 181 and CSF levels of Aβ, total Tau, and p tau.
Patient characteristic
In this study, baseline cohort information of 21 patients diagnosed with Alzheimer's was entered. The mean age was 74.9 years, with a minimum of 61 years and a maximum of 90 years, and included 14 (66.7%) men and 7 (33.3%) women. Patients were all educated, and the average length of the education was 16.7 years. The mean time interval between diagnosis and study time is 2.475 years, and the mean scores of the MMSE test were 23.3, and CDR was 0.78 (Tab1).
Comprehensive Analysis
The single linear regression results showed that age is not a predictor for CSF biomarkers levels, and there is no correlation between age and p tau 181 plasma level and MMSE score. Education level is not related to other variables, and the
mean time interval between diagnosis and study time is not related to CSF and plasma biomarkers.
Linear regression analysis for each revealed that the amount of total tau protein and phosphorylated tau protein in CSF was not related to MMSE score, hippocampal, fusiform, white matter hyperintensity, white matter, and gray matter volume and also do not have the ability to predict the serum level of p tau 181. Nevertheless, there was a significant relationship between p tau and t tau levels in CSF (p <0.05).
Amyloid β levels in CSF correlated with white matter volume (p <0.05), and this biomarker is a strong predictor of plasma levels of p tau 181 (p <0.05). However, also no relationship was observed between this biomarker and other CSF biomarkers.
CSF biomarkers and microstructural changes
Uncontrolled linear regression by adding Amyloid β as an independent variable and DTI values in each ROI as a dependent variable showed that the amyloid β level associated with changes in AD, RD, MD, and FA in the anterior corona radiate, hippocampal cingulum, fronto-occipital fasciculus, sagittal stratum (p <0.05).
T tau is also associated with changes in the body of corpus callosum, posterior corona radiate, posterior limb of the internal capsule, retrolenticular part of internal capsule, splenium of corpus callosum, superior corona radiata, tapetum, superior fronto occipital fasciculus, and anterior limb of internal capsule (p <0.05).
Furthermore, the results of the similar analysis for p tau also indicate that there is an association between CSF p tau and imaging parameters in corpus callosum, posterior corona radiate, posterior limb of internal capsule, posterior thalamic radiation, retrolenticular part of internal capsule, superior corona radiata, superior fronto-occipital fasciculus, Anterior limb of internal capsule (p <0.05).
Plasma p tau 181 and microstructural changes
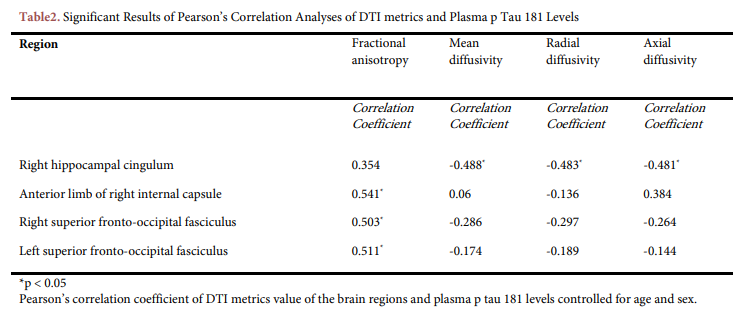
To investigate white matter pathways related to plasma p tau 181, we created multiple linear regression models, including age, sex, and p tau 181. The analysis revealed that the plasma level of p tau 181 could predict changes of MD, RD, AD parameters in the right hippocampal cingulum (p <0.05), and there is an association between its level and FA change in the anterior limb of the right internal capsule and bilateral superior fronto-occipital fasciculus. In the next step to confirm the DTI connectometry approach results, we investigated the relationship between p tau 181 and microstructural changes in the mentioned areas in using partial correlation controlled for the effect of age and sex (Tab2). A significant negative relationship was observed between p tau 181 and MD (Pearson's coefficient = -0.488), RD (Pearson's coefficient = -0.483), and AD (Pearson's coefficient = -0.481) in the right hippocampal cingulum. P tau 181 is also positively correlated with changes of FA in the anterior limb of the right internal capsule (Pearson's coefficient = 0.541), right superior fronto-occipital fasciculus (Pearson's coefficient = 0.503), and left superior fronto-occipital fasciculus (Pearson's coefficient = 0.511).
Discussion
In a cross-sectional study based on the ADNI cohort, we concluded that the plasma level of p tau 181 independently predicts microstructural changes in the brain of Alzheimer's patients. We used a step-by-step strategy; first, we investigate the relationship between CSF biomarkers, plasma p tau 181, and patient characteristics. Then we narrowed the study to examine the correlation between CSF biomarkers and plasma p tau 181 with DTI connectometry values in each region. Then we used a partial correlation model controlled for age and sex to confirm the results.
Baseline plasma p tau 181 levels are associated with regional white matter changes in AD patients in the disease's pathological areas, including the right hippocampal cingulum, anterior limb of the internal capsule, and bilateral superior fronto-occipital fasciculus. Our study results about CSF biomarkers' correlations are in line with Claudia Drummond et al. study, which shows the negative correlation between CSF biomarkers and MD values changes in the Uncifasciculusateral parahippocampal cingulate and occipitofrontal fasciculus (28). Similarly, X Li et al. Found that pathological levels of Aβ42 and CSF total tau in people with Alzheimer's-related cognitive impairments correlated with decreased FA and increased MD in the white matter pathway (29). Although CSF tau and plasma p tau 181 have a significant overlap in predicting changes in the fronto-occipital fasciculus and anterior limb of the right internal capsule. Nevertheless, unlike plasma p tau 181, CSF tau had no significant association with changes in the hippocampal cingulum. The absence of correlation between CSF tau and cingulum contradicts the results of Vidar Stenset et al., which identified CSF tau as a predictor of FA and RD changes in the cingulum (30). On the other hand, plasma tau concentration is associated with several memory-related structures in the medial temporal lobe, including the parahippocampus and hippocampus (31). The lack of correlation between CSF biomarkers and hippocampal volume reduction in our study confirms the Falcon study's findings, which also found no correlation (32).
In the onset of dementia and cognitive decline, many areas seem to change. The internal capsule, fronto-occipital fasciculus, and hippocampal cingulum in AD people change compared to healthy people without cognitive problems (33). In our study, plasma p tau 181 levels and CSF biomarkers were significantly associated with these areas, indicating that our findings are in line with previous studies investigating microstructural changes related to Alzheimer's. As results of our analysis, plasma p tau 181 mostly predicted structural changes in the right hippocampal cingulum, which indicate the importance of the cingulum as an important area in the pathological course of the disease, and there is also evidence of a link between CSF p tau and Aβ with a change of MD in the cingulum region (34). Moreover, the research results provide compelling evidence in support of our findings and state that brain connectivity in the posterior cingulum can be a good predictor for cognitive decline in Alzheimer's disease (35). The cingulum bundle is an important white matter tract that connects the frontal, parietal, and medial temporal, linking the subcortical nucleus to the cingulate gyrus and extending into the hippocampal and parahippocampal regions, and for that damage in areas close to the hippocampus in the cingulum causes cognitive problems in many domains such as language, memory and executive control (36).
Another notable finding of our study was the absence of association between CSF biomarkers and serum levels of p tau 181, which was similar to Fossati et al. result that showed plasma tau levels were independent of CSF levels and the reason for this finding was the measurement of different biological compartments of tau (37).
Biomarkers in CSF may be active several years before the onset of symptoms, and Aβ and Tau are most critical (38). Many studies have emphasized the diagnostic role of t tau and p tau in CSF and state that they can predict the progression to dementia (39-42). On the other hand, some studies present different findings (43, 44). Despite this, in predicting AD by each of these biomarkers alone, p-tau preferred in terms of specificity and sensitivity (38).
Studies have been conducted on plasma biomarkers in recent years, and there is great hope for it. Evidence revealed that plasma p tau 181 and t tau levels have a high diagnostic value, and their levels are much higher in AD subjects than in MCI and healthy control (45). However, studies have described p tau 181 better than t tau (9, 10). The largest plasma p tau 181 study in the diagnosis of Alzheimer's, which included the results of four independent cohorts, states that plasma p tau 181 has a high performance in identifying the clinical diagnosis of Alzheimer's patients with an unknown amyloid status and was able to differentiate Alzheimer's disease from other neurodegenerative diseases and with Aβ PET can detect Alzheimer's in the early stages (11).
In conclusion, our study results show that plasma p tau 181 levels are associated with microstructural changes in pathogenesis areas of Alzheimer's disease, which enhance this biomarker's diagnostic status. Due to the increasing population with Alzheimer's and the resulting social costs and considering that AD's pathogenesis exists several years before its clinical signs, achieving a reliable biomarker with adequate sensitivity and specificity is necessary. Although plasma p tau 181 is superior to CSF biomarkers and imaging techniques in terms of availability, low cost, and non-invasiveness. More efforts should be made to standardize biomarkers' measurement and define their pathological threshold. Longitudinal studies are also necessary to prove the efficacy of these biomarkers predicting role in structural changes.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson &
Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Declarations
Funding
We do not have any financial support for this study.
Conflict of interest
The authors have no conflicts of interest to disclose.
Availability of data
The datasets analyzed during the current study are available upon request with no restriction.
Code availability
Not applicable
Authors' contributions
FN and MP came up with the idea for the paper, FN, SK, and AR drafted it, and FN, MP, FR, and ND helped to write it. All of the authors reviewed the article.
Ethical approval
The data in this paper were obtained from the ADNI database (adni.loni.usc.edu). It does not include any examination of human or animal subjects.
Consent for publication
This manuscript has been approved for publication by all authors.
| 1. Mantzavinos V, Alexiou A. Biomarkers for Alzheimer's Disease Diagnosis. Curr Alzheimer Res. 2017;14(11):1149-54. https://doi.org/10.2174/1567205014666170203125942 PMid:28164766 PMCid:PMC5684784 |
||||
| 2. Oboudiyat C, Glazer H, Seifan A, Greer C, Isaacson RS. Alzheimer's disease. Semin Neurol. 2013;33(4):313-29. https://doi.org/10.1055/s-0033-1359319 PMid:24234352 |
||||
| 3. Weller J, Budson A. Current understanding of Alzheimer's disease diagnosis and treatment. F1000Res. 2018;7:F1000 Faculty Rev-161. https://doi.org/10.12688/f1000research.14506.1 PMid:30135715 PMCid:PMC6073093 |
||||
| 4. Wu XL, Piña-Crespo J, Zhang YW, Chen XC, Xu HX. Tau-mediated Neurodegeneration and Potential Implications in Diagnosis and Treatment of Alzheimer's Disease. Chin Med J (Engl). 2017;130(24):2978-90. https://doi.org/10.4103/0366-6999.220313 PMid:29237931 PMCid:PMC5742926 |
||||
| 5. Kandimalla R, Manczak M, Yin X, Wang R, Reddy PH. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum Mol Genet. 2018;27(1):30-40. https://doi.org/10.1093/hmg/ddx381 PMid:29040533 PMCid:PMC5886218 |
||||
| 6. Rissman RA. Stress-induced tau phosphorylation: functional neuroplasticity or neuronal vulnerability? J Alzheimers Dis. 2009;18(2):453-7. https://doi.org/10.3233/JAD-2009-1153 PMid:19584431 PMCid:PMC2906152 |
||||
| 7. Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer's disease. Exp Gerontol. 2010;45(1):30-40. https://doi.org/10.1016/j.exger.2009.10.010 PMid:19853650 PMCid:PMC2815003 |
||||
| 8. Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673-84. https://doi.org/10.1016/S1474-4422(16)00070-3 PMid:27068280 |
||||
| 9. Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer's disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 2018;14(8):989-97. https://doi.org/10.1016/j.jalz.2018.02.013 PMid:29626426 PMCid:PMC6097897 |
||||
| 10. Yang CC, Chiu MJ, Chen TF, Chang HL, Liu BH, Yang SY. Assay of Plasma Phosphorylated Tau Protein (Threonine 181) and Total Tau Protein in Early-Stage Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2018;61(4):1323-32. https://doi.org/10.3233/JAD-170810 PMid:29376870 |
||||
| 11. Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. The Lancet Neurology. 2020;19(5):422-33. https://doi.org/10.1016/S1474-4422(20)30071-5 PMid:32333900 |
||||
| 12. Nabizadeh F, Pourhamzeh M, Khani S, Rezaei A, Ranjbaran F, Deravi N, et al. Plasma phosphorylated-tau181 levels reflect white matter microstructural changes across Alzheimer's disease progression. Metabolic Brain Disease. 2022;37(3):761-71. https://doi.org/10.1007/s11011-022-00908-7 PMid:35015198 |
||||
| 13. Nabizadeh F. What should we do to reduce the complications of Deep brain stimulation in Parkinson's disease? Neurology Letters. 2022;1(1, Continuous):1-11. https://doi.org/10.52547/nl.1.1.1 |
||||
| 14. Nabizadeh F, Balabandian M, Rostami MR, Kankam SB, Ranjbaran F, Pourhamzeh M, et al. Plasma neurofilament light levels correlate with white matter damage prior to Alzheimer's disease: results from ADNI. Aging Clinical and Experimental Research. 2022. https://doi.org/10.1007/s40520-022-02095-y PMid:35226303 |
||||
| 15. Nabizadeh F, Balabandian M, Rostami MR, Ward RT, Ahmadi N, Pourhamzeh M, et al. Plasma p-tau181 associated with structural changes in mild cognitive impairment. Aging Clinical and Experimental Research. 2022. https://doi.org/10.1007/s40520-022-02148-2 PMid:35648357 |
||||
| 16. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939-44. https://doi.org/10.1212/WNL.34.7.939 PMid:6610841 |
||||
| 17. Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403-13. https://doi.org/10.1002/ana.21610 PMid:19296504 PMCid:PMC2696350 |
||||
| 18. Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg Br, et al. Simultaneous Measurement of β-Amyloid(1-42), Total Tau, and Phosphorylated Tau (Thr181) in Cerebrospinal Fluid by the xMAP Technology. Clinical Chemistry. 2005;51(2):336-45. https://doi.org/10.1373/clinchem.2004.039347 PMid:15563479 |
||||
| 19. Iglesias JE, Liu CY, Thompson PM, Tu Z. Robust brain extraction across datasets and comparison with publicly available methods. IEEE Trans Med Imaging. 2011;30(9):1617-34. https://doi.org/10.1109/TMI.2011.2138152 PMid:21880566 |
||||
| 20. Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11-22. https://doi.org/10.1093/cercor/bhg087 PMid:14654453 |
||||
| 21. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143-55. https://doi.org/10.1002/hbm.10062 PMid:12391568 PMCid:PMC6871816 |
||||
| 22. Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22(2):324-33. https://doi.org/10.1097/00004728-199803000-00032 PMid:9530404 |
||||
| 23. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825-41. https://doi.org/10.1006/nimg.2002.1132 PMid:12377157 |
||||
| 24. Leow AD, Yanovsky I, Chiang MC, Lee AD, Klunder AD, Lu A, et al. Statistical properties of Jacobian maps and the realization of unbiased large-deformation nonlinear image registration. IEEE Trans Med Imaging. 2007;26(6):822-32. https://doi.org/10.1109/TMI.2007.892646 PMid:17679333 |
||||
| 25. Jahanshad N, Lee AD, Barysheva M, McMahon KL, de Zubicaray GI, Martin NG, et al. Genetic influences on brain asymmetry: a DTI study of 374 twins and siblings. Neuroimage. 2010;52(2):455-69. https://doi.org/10.1016/j.neuroimage.2010.04.236 PMid:20430102 PMCid:PMC3086641 |
||||
| 26. Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570-82. https://doi.org/10.1016/j.neuroimage.2007.12.035 PMid:18255316 PMCid:PMC2478641 |
||||
| 27. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487-505. https://doi.org/10.1016/j.neuroimage.2006.02.024 PMid:16624579 |
||||
| 28. Drummond C, Coutinho G, Monteiro MC, Assuncao N, Teldeschi A, de Souza AS, et al. Narrative impairment, white matter damage and CSF biomarkers in the Alzheimer's disease spectrum. Aging. 2019;11(20):9188-208. https://doi.org/10.18632/aging.102391 PMid:31682234 PMCid:PMC6834410 |
||||
| 29. Li X, Li TQ, Andreasen N, Wiberg MK, Westman E, Wahlund LO. The association between biomarkers in cerebrospinal fluid and structural changes in the brain in patients with Alzheimer's disease. Journal of internal medicine. 2014;275(4):418-27. https://doi.org/10.1111/joim.12164 PMid:24237038 |
||||
| 30. Stenset V, Bjørnerud A, Fjell AM, Walhovd KB, Hofoss D, Due-Tønnessen P, et al. Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiology of aging. 2011;32(4):581-9. https://doi.org/10.1016/j.neurobiolaging.2009.04.014 PMid:19428143 |
||||
| 31. Deters KD, Risacher SL, Kim S, Nho K, West JD, Blennow K, et al. Plasma Tau Association with Brain Atrophy in Mild Cognitive Impairment and Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2017;58(4):1245-54. https://doi.org/10.3233/JAD-161114 PMid:28550246 PMCid:PMC5523909 |
||||
| 32. Falcon C, Tucholka A, Monté-Rubio GC, Cacciaglia R, Operto G, Rami L, et al. Longitudinal structural cerebral changes related to core CSF biomarkers in preclinical Alzheimer's disease: A study of two independent datasets. NeuroImage: Clinical. 2018;19:190-201. https://doi.org/10.1016/j.nicl.2018.04.016 PMid:30023169 PMCid:PMC6050455 |
||||
| 33. Mayo CD, Mazerolle EL, Ritchie L, Fisk JD, Gawryluk JR. Longitudinal changes in microstructural white matter metrics in Alzheimer's disease. NeuroImage Clinical. 2017;13:330-8. https://doi.org/10.1016/j.nicl.2016.12.012 PMid:28066707 PMCid:PMC5200876 |
||||
| 34. Racine AM, Merluzzi AP, Adluru N, Norton D, Koscik RL, Clark LR, et al. Association of longitudinal white matter degeneration and cerebrospinal fluid biomarkers of neurodegeneration, inflammation and Alzheimer's disease in late-middle-aged adults. Brain Imaging Behav. 2019;13(1):41-52. https://doi.org/10.1007/s11682-017-9732-9 PMid:28600739 PMCid:PMC5723250 |
||||
| 35. Nakata Y, Sato N, Nemoto K, Abe O, Shikakura S, Arima K, et al. Diffusion abnormality in the posterior cingulum and hippocampal volume: correlation with disease progression in Alzheimer's disease. Magnetic resonance imaging. 2009;27(3):347-54. https://doi.org/10.1016/j.mri.2008.07.013 PMid:18771871 |
||||
| 36. Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience & Biobehavioral Reviews. 2018;92:104-27. https://doi.org/10.1016/j.neubiorev.2018.05.008 PMid:29753752 PMCid:PMC6090091 |
||||
| 37. Fossati S, Ramos Cejudo J, Debure L, Pirraglia E, Sone JY, Li Y, et al. Plasma tau complements CSF tau and P-tau in the diagnosis of Alzheimer's disease. Alzheimers Dement (Amst). 2019;11:483-92. https://doi.org/10.1016/j.dadm.2019.05.001 PMid:31334328 PMCid:PMC6624242 |
||||
| 38. Tan CC, Yu JT, Tan L. Biomarkers for preclinical Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2014;42(4):1051-69. https://doi.org/10.3233/JAD-140843 PMid:25024325 |
||||
| 39. Lewczuk P, Lelental N, Lachmann I, Holzer M, Flach K, Brandner S, et al. Non-Phosphorylated Tau as a Potential Biomarker of Alzheimer's Disease: Analytical and Diagnostic Characterization. Journal of Alzheimer's disease : JAD. 2017;55(1):159-70. https://doi.org/10.3233/JAD-160448 PMid:27662295 |
||||
| 40. Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer's disease. Molecular neurobiology. 2001;24(1-3):87-97. https://doi.org/10.1385/MN:24:1-3:087 PMid:11831556 |
||||
| 41. Schönknecht P, Pantel J, Hunt A, Volkmann M, Buerger K, Hampel H, et al. Levels of total tau and tau protein phosphorylated at threonine 181 in patients with incipient and manifest Alzheimer's disease. Neuroscience letters. 2003;339(2):172-4. https://doi.org/10.1016/S0304-3940(02)01481-7 PMid:12614922 |
||||
| 42. Lewczuk P, Esselmann H, Bibl M, Beck G, Maler JM, Otto M, et al. Tau protein phosphorylated at threonine 181 in CSF as a neurochemical biomarker in Alzheimer's disease: original data and review of the literature. Journal of molecular neuroscience : MN. 2004;23(1-2):115-22. https://doi.org/10.1385/JMN:23:1-2:115 PMid:15126697 |
||||
| 43. Rizzi L, Missiaggia L, Roriz-Cruz M. CSF Aβ(1-42), but not p-Tau(181), Predicted Progression from Amnestic MCI to Alzheimer's Disease Dementia. Neuromolecular medicine. 2018;20(4):491-7. https://doi.org/10.1007/s12017-018-8516-8 PMid:30306396 |
||||
| 44. Haense C, Buerger K, Kalbe E, Drzezga A, Teipel SJ, Markiewicz P, et al. CSF total and phosphorylated tau protein, regional glucose metabolism and dementia severity in Alzheimer's disease. European journal of neurology. 2008;15(11):1155-62. https://doi.org/10.1111/j.1468-1331.2008.02274.x PMid:18803648 |
||||
| 45. Shekhar S, Kumar R, Rai N, Kumar V, Singh K, Upadhyay AD, et al. Estimation of Tau and Phosphorylated Tau181 in Serum of Alzheimer's Disease and Mild Cognitive Impairment Patients. PLoS One. 2016;11(7):e0159099-e. https://doi.org/10.1371/journal.pone.0159099 PMid:27459603 PMCid:PMC4961391 |
||||
Volume 1, Issue 1
June 2022Pages 21-27
- Receive Date: 09 June 2022
- Revise Date: 17 June 2022
- Accept Date: 16 June 2022
 Neurology Letters
Neurology Letters
